Day 1 :
Keynote Forum
M Raj Lakshman
VA Medical Center, USA
Keynote: Some novel modulators of obesity and atherogenic factors
Time : 10:00-10:45

Biography:
Abstract:
Keynote Forum
M Raj Lakshman
VA Medical Center, USA
Keynote: Some novel modulators of obesity and atherogenic factors
Time : 10:00-10:45

Biography:
Abstract:
- Lipids in Molecular Medicine | Lipid and Lipoprotein Metabolism | Protein-Lipid and Lipid-Lipid Interactions | Techniques Involved in Lipid Research | Lipidomics - What’s next?
Location: Olimpica 3

Chair
M Raj Lakshman
VA Medical Center, USA
Session Introduction
Jingwen Liu
Veterans Affairs Palo Alto Health Care System, USA
Title: FXR activation by obeticholic acid induces hepatic SR-BI gene expression through a novel mechanism of transcriptional synergy with the nuclear receptor LXR
Time : 11:05-11:35

Biography:
Abstract:
 The farnesoid X receptor (FXR) and liver X receptor (LXR) are known to regulate distinct biological pathways in BA synthesis and cholesterol metabolism. Obeticholic acid (OCA) is an FXR agonist being developed for treating various chronic liver diseases. Previous studies reported inconsistent effects of OCA on regulating plasma cholesterol levels in different animal models and in different patient populations. The mechanisms underlying its divergent effects yet have not been thoroughly investigated. The scavenger receptor class B type I (SR-BI) is an FXR modulated gene and the major receptor for HDL-C. We recently showed that OCA treatment effectively lowered plasma HDL-C levels and increased hepatic SR-BI expression in hypercholesterolemic hamsters but not in normolipidemic hamsters, suggesting that hepatic cholesterol might play a role in OCA-induced SR-BI transcription. In this current study, by conducting genomic sequence analysis, reporter assays and direct DNA binding assays, we have identified a highly conserved regulatory region in the first intron of hamster SR-BI gene that contains a functional FXRE motif and an LXRE site separated by 57 base pairs. Promoter reporter activity assays demonstrated the functional involvement of this critical regulatory region in SR-BI gene transcription upon activations of FXR and LXR in a synergistic fashion. In vivo studies of normolipidemic hamsters showed that hepatic SR-BI gene expression is not affected by separate treatment of OCA or LXR agonist GW3965 but the co-activation of FXR and LXR by the combined treatment of OCA and GW3965 results in significant increases in hepatic SR-BI mRNA and protein levels. Taken together, we have discovered an unprecedented cross-talk between FXR and LXR that leads to a synergistic activation of SR-BI gene transcription in liver tissue and consequently affected HDL-C metabolism. Our novel findings shed new light for a better understanding of lipid regulatory effects of OCA in hyperlipidemic patients.
The farnesoid X receptor (FXR) and liver X receptor (LXR) are known to regulate distinct biological pathways in BA synthesis and cholesterol metabolism. Obeticholic acid (OCA) is an FXR agonist being developed for treating various chronic liver diseases. Previous studies reported inconsistent effects of OCA on regulating plasma cholesterol levels in different animal models and in different patient populations. The mechanisms underlying its divergent effects yet have not been thoroughly investigated. The scavenger receptor class B type I (SR-BI) is an FXR modulated gene and the major receptor for HDL-C. We recently showed that OCA treatment effectively lowered plasma HDL-C levels and increased hepatic SR-BI expression in hypercholesterolemic hamsters but not in normolipidemic hamsters, suggesting that hepatic cholesterol might play a role in OCA-induced SR-BI transcription. In this current study, by conducting genomic sequence analysis, reporter assays and direct DNA binding assays, we have identified a highly conserved regulatory region in the first intron of hamster SR-BI gene that contains a functional FXRE motif and an LXRE site separated by 57 base pairs. Promoter reporter activity assays demonstrated the functional involvement of this critical regulatory region in SR-BI gene transcription upon activations of FXR and LXR in a synergistic fashion. In vivo studies of normolipidemic hamsters showed that hepatic SR-BI gene expression is not affected by separate treatment of OCA or LXR agonist GW3965 but the co-activation of FXR and LXR by the combined treatment of OCA and GW3965 results in significant increases in hepatic SR-BI mRNA and protein levels. Taken together, we have discovered an unprecedented cross-talk between FXR and LXR that leads to a synergistic activation of SR-BI gene transcription in liver tissue and consequently affected HDL-C metabolism. Our novel findings shed new light for a better understanding of lipid regulatory effects of OCA in hyperlipidemic patients.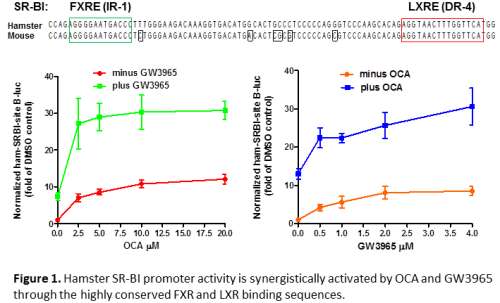
Charles Chalfant
Virginia Commonwealth University, USA
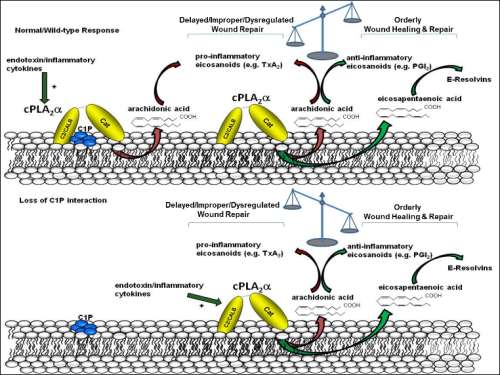
Title: Ablation of the ceramide-1-phosphate interaction with group IVA cytosolic phospholipase A2 induces enhanced wound regenerations
Time : 11:35-12:05

Biography:
Abstract:
Giuseppe Maulucci
Università Cattolica del Sacro Cuore, Italy
Title: Quantitative analysis of fatty acid metabolism by confocal spectral imaging of intracellular polarity
Time : 12:05-12:35

Biography:
Abstract:

Chryssostomos Chatgilialoglu
Consiglio Nazionale delle Ricerche, Italy
Title: Fatty acid-based membrane lipidomics: Connections with metabolic and nutritional issue
Time : 12:35-13:05

Biography:
Abstract:
Calvano Cosima Damiana
Università di Bari, Italy
Title: Development and use of advanced mass spectrometry techniques for the characterization of cellular lipidome profile of fibroblasts in early on-set Parkinson’s disease patients
Time : 14:05-14:35

Biography:
Abstract:
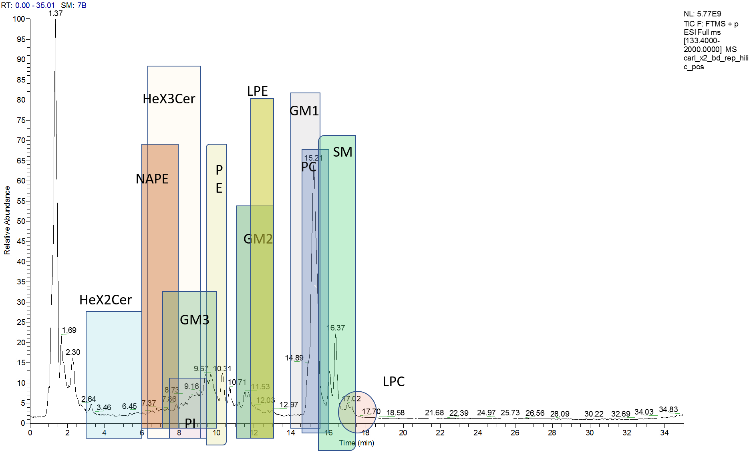
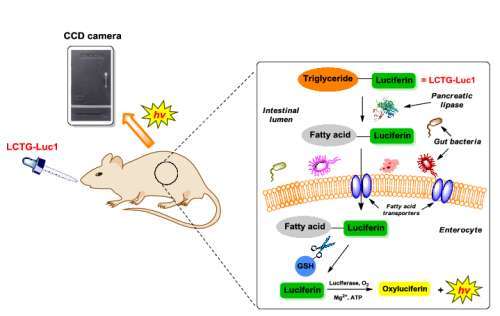
Elena A Goun
École Polytechnique Fédérale de Lausanne, Switzerland
Title: Novel optical real-time imaging tools reveal large effects of gut microbiota on lipid uptake in live animals
Time : 14:35-15:05

Biography:
Abstract:
Sergey Korolev
Saint Louis University School of Medicine, USA
Title: Mechanism of phospholipase A2 G6A activity and regulation revealed by the novel crystal structure
Time : 15:05-15:35

Biography:
Abstract: