
Charles Chalfant
Virginia Commonwealth University, USA
Title: Ablation of the ceramide-1-phosphate interaction with group IVA cytosolic phospholipase A2 induces enhanced wound regenerations
Biography
Biography: Charles Chalfant
Abstract
New roles for sphingolipids such as ceramide, ceramide-1-phosphate (C1P), and sphingosine-1-phosphate continue to emerge. My research, for example, has implicated C1P as a major regulator of eicosanoid synthesis, and despite the importance of eicosanoids in the inflammatory process, the regulation of eicosanoid synthesis proximal to the activation of Group IVA phospholipase A2 (cPLA2α) is still an enigma. In this regard, my laboratory demonstrated that C1P is a direct and required lipid co-factor for cPLA2α activation in cellular models. In further studies, one interaction site for C1P was localized to the calcium-lipid binding domain (C2 domain) of the enzyme allowing for the genetic ablation of the site in vivo via the generation of a cPLA2α knock-in (KI) mouse. In this lecture, the characterization of this new mouse model in comparison to the full genetic ablation of the enzyme will be presented. Specifically, the loss of the C1P/cPLA2α interaction induced a class-switch in the production specific eicosanoids and specialized lipid mediators driving accelerated wound repair and regeneration, both in acute and chronic murine models. Cellular studies demonstrated that loss of this lipid, protein interaction led to enhanced dermal fibroblast and neutrophil migration, which was mimicked in vivo. In further mechanistic studies, C1P was found to modulate the substrate specificity of cPLA2α in opposition to another lipid mediator of the enzyme, PIP2, explaining the class switch as to bioactive lipid mediators observed in the cPLA2α KI mouse. Using lipidomic analyses, these specific lipid fingerprints were linked to human wound healing outcomes, which suggests that modulation of specific lipid mediators could be explored to promote wound healing and regeneration in a number of contexts.
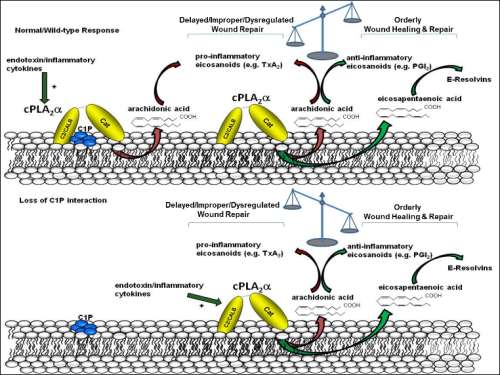
Figure 1.The current mechanistic hypothesis, “shifting function hypothesis, to explain the loss of pro-inflammatory lipid mediators and concomitant increase in pro-resolution lipid mediators in response to loss of the C1P/cPLA2α interaction.

